An international research team around cell biologist Dr. Jennifer Schoberer from the University of Natural Resources and Applied Life Sciences, Vienna (BOKU) and Prof. Stan Botchway from the Central Laser Facility, STFC, describes in the current issue of "Nature Communications" how plant cells protect themselves against an arbitrary degradation of glycoproteins. During the experiment, the team discovered a previously unknown sugar modification protein relating to plant hardiness which could potentially lead to plants that are more robust to changing environmental conditions, thus securing global crop production
 The biosynthesis and modification of protein-bound sugar structures, the so-called protein glycosylation, is one of the most common forms of protein modification. Nearly 50% of all eukaryotic proteins are glycosylated. The bound sugar structures (glycans) influence numerous biological processes and are therefore essential for the development of organisms as well as their adaptation to changing environmental conditions.
The biosynthesis and modification of protein-bound sugar structures, the so-called protein glycosylation, is one of the most common forms of protein modification. Nearly 50% of all eukaryotic proteins are glycosylated. The bound sugar structures (glycans) influence numerous biological processes and are therefore essential for the development of organisms as well as their adaptation to changing environmental conditions.
In a specific compartment of the cell called the endoplasmic reticulum (ER), certain glycans play a critical role in the folding of proteins. This vital cellular process can result in the formation of incompletely folded or defective proteins, which must be removed to maintain the cell's balance and prevent a possible cell death.
Cells have therefore developed a complex quality control system and a specific protein degradation process that prevents the accumulation of altered ("harmful") proteins. To detect and sort out incorrectly folded glycoproteins, a sugar signal is required, which is formed by certain enzymes, so-called mannosidases, in the ER. Mannosidases are also involved in the processing of other important sugar structures in the ER and Golgi apparatus. To ensure a controlled course of these events in the cell, the involved mannosidases must be spatially separated.
In the present work, a previously unknown signal was discovered, which leads to a strict spatial separation of the involved mannosidase MNS3 in the Golgi apparatus and thus prevents the arbitrary (as opposed to deliberately controlled) degradation of glycoproteins in the ER. This discovery, if utilised, could potentially create crops that are hardier to a changing environment.
This research was supported by the Austrian Science Fund (FWF) and the STFC UK.
Publication online: https://www.nature.com/articles/s41467-019-11686-9
doi: 10.1038/s41467-019-11686-9
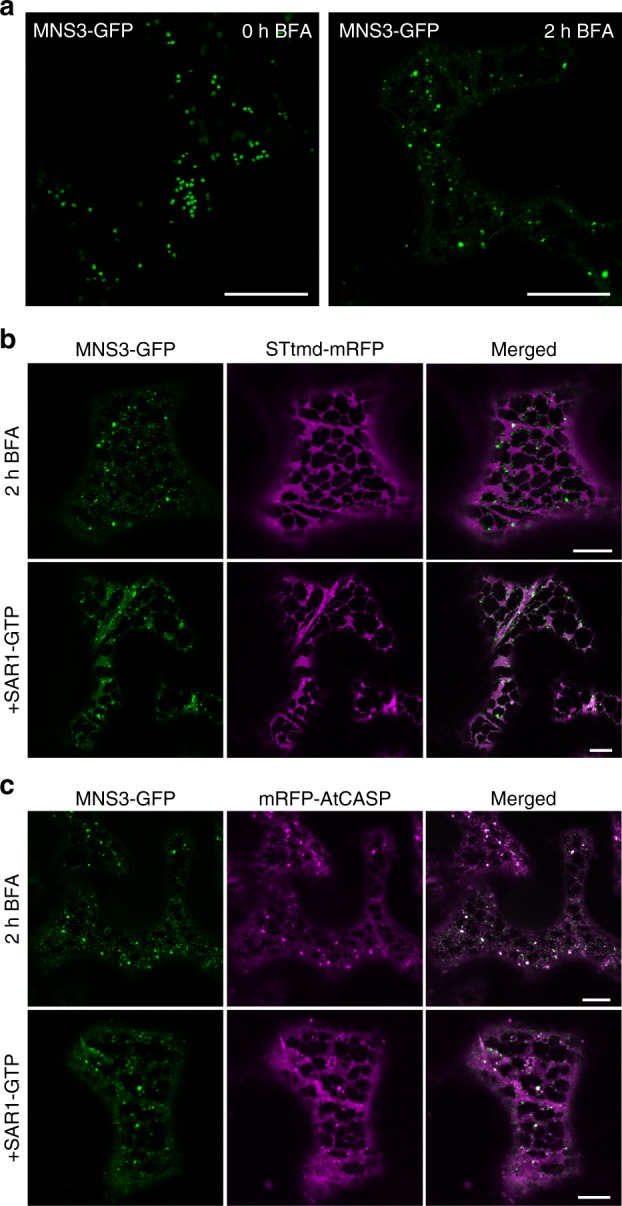
Fig 1: MNS3 largely remains on punctate structures during Golgi disassembly. Fusion proteins were transiently expressed in N. benthamiana leaf epidermal cells and observed 2–3 dpi using confocal microscopy. a Confocal images showing the subcellular localization of MNS3-GFP before (0 h BFA) and after BFA addition (2 h BFA). Scale bars = 20 µm. b A representative cell that co-expresses MNS3-GFP (green) with STtmd-mRFP (magenta) and was treated with BFA for 2 h (upper panel) or additionally expresses GTP-locked SAR1 (SAR1-GTP, lower panel). Scale bars = 10 µm. c A representative cell that co-expresses MNS3-GFP (green) with the cis-Golgi golgin mRFP-AtCASP (magenta) and was treated with BFA for 2 h (upper panel) or additionally expresses SAR1-GTP (lower panel). Scale bars = 10 µm
[BS(1]
[BS(1]Why deleted?
