Collaborators from the University of York and the Central Laser Facility have been looking into measuring proteins in H2O using 2D-IR spectroscopy at our Ultra Laser facility. The methods from this experiment could potentially be applied to the analysis of proteins in biofluids such as blood serum, leading to the earlier diagnosis of diseases.
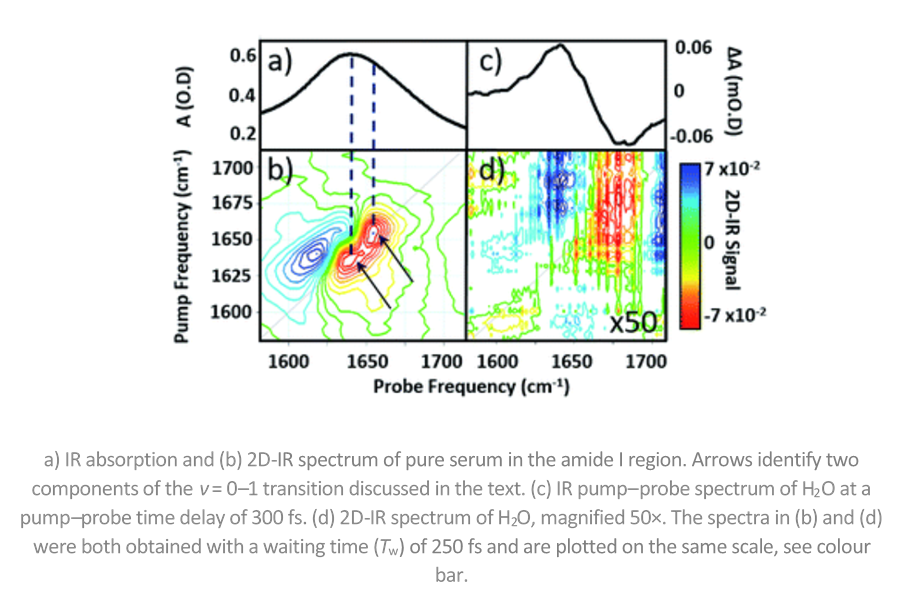
The tricky thing about looking at proteins in H2O is that water absorbs IR light at the same wavelength as proteins. Infrared spectroscopy is sensitive to molecular structure, but its application to proteins is hindered by the broad nature of the amide I band, essentially the C=O stretching mode of the peptide link.
However, the team led by Prof. Neil Hunt overcame this problem by using 2D-IR spectroscopy. 2D-IR measures vibrational couplings between peptide units in macromolecular structures and the 2D-IR amide I signal is diagnostic of secondary structure content and ligand binding. Importantly, the team were able to show that 2D-IR amide I signature of proteins dominates that of water even at sub-millimolar protein concentrations.
This week, the paper was selected for “Pick of the Week" by Chemical Science, the flagship journal of the Royal Society of Chemistry.
Professor Hunt explains the experiment in the ChemSci article, using a clever analogy: "Imagine a protein molecule in water as being like a needle in a haystack – a small number of proteins (needles) are surrounded by a large number of water molecules (hay) that obscure our view of the proteins. The 2D-IR method makes the signal from the protein many times stronger than that from the water – the hay is effectively made transparent, so we can see the needles clearly."
"With further development work 2D-IR could become a new method for blood sample analysis. An alternative future direction could see 2D-IR being used in drug design projects. 2D-IR is a powerful tool for studying protein-drug binding but currently scientists need to replace H2O with expensive D2O to use 2D-IR. Our method removes that need, making the application of information-rich 2D-IR spectroscopy to protein-drug systems much more economical, straightforward and so accessible."
The “Pick of the Week" article, which contains a fantastic infographic, can be found here: https://www.rsc.org/news-events/journals-highlights/2019/jun/needle-in-a-haystack/
The original paper can be found here: https://doi.org/10.1039/C9SC01590F