Their findings are reported in the prestigious journal Nature Chemistry.
Harnessing the unique capabilities of the world-class CLF ULTRA facility, the group were able to beautifully illustrate the interplay between chromophore dynamics and surrounding amino-acid residues, an interaction that underlies the process of fluorescent protein photochromism.
The team, led by Professor Peter Tonge from Stony Brook University in New York and Professor Steve Meech from University of East Anglia, looked at a prototypical photochromic fluorescent protein called dronpa. Fluorescent proteins are special types of proteins that possess a chemical compound called a fluorophore that can re-emit light following excitation. This is handy in nature because it allows for bioluminescence, a kind of biological torch to attract mates or scare off predators. Photochromic fluorescence, however, is subtly different. It describes the process by which proteins undergo a reversible colour change when they are exposed to varying frequencies of light. As illustrated below (Figure 1), Dronpa's light switch can be turned on or off; if blue light of 450nm is absorbed by its fluorophore then it remains in an 'on' state. The protein's luminescence factory can then be turned off by stimulation with a UV wavelength of 400nm.
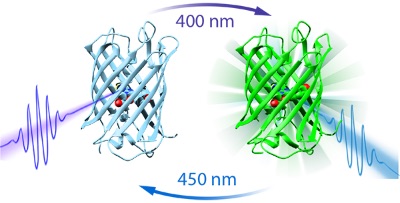
Figure 1: A diagram showing how the dronpa light switch can be switched on or off depending on the colour of light used to stimulate it (either 400nm or 450nm). Image credit, Stony Brook University.
Whilst scientists have previously highlighted the role of trans-to-cis isomerization and proton transfer in dronpa photo activation , studies in this field have usually been limited by factors such as spectral resolution with regards to electronic transitions, or the accessible time range when it comes to traditional infrared and x-ray experiments. It is for these reasons that researchers have for years struggled to grapple the concept of x and unravel the actual mechanism by which dronpa undergoes it's off to on state conversion. This is where the unique ultrafast time resolved infrared capabilities of the Central Laser Facilities' Ultra laser come in.
Using high signal – to - noise femtosecond to millisecond transient infrared difference (TRIR) spectroscopy on Ultra, the group were able to not only resolve the primary photochemical step in the on-off transitionary process, but also identify a series of ground-state structural transformations (Figure 2).
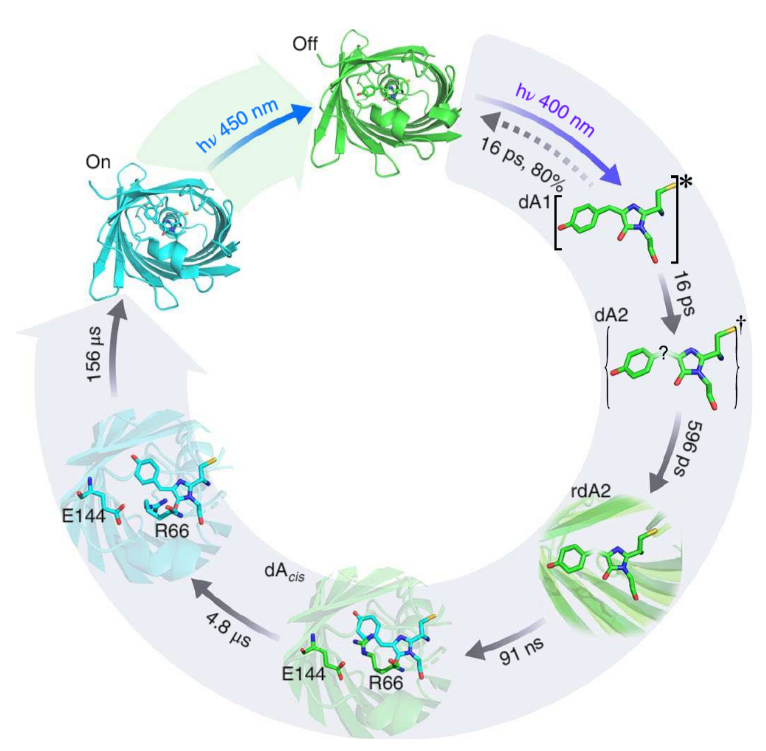
Figure 2: (Laptenok et al, 2018)
“A key challenge in understanding how the switch works in dronpa is to determine how the initial interaction of light — which happens extremely fast, in less than one quadrillionth of a second — changes the dynamics and ultimately turns the switch on in a process that occurs millions of times more slowly. In our work we used an instrument that can look at the vibrations of dronpa over many decades of time so that we could visualize the entire activation process in one experiment."
Professor Peter Tonge (Stony Brook University)
The authors are hopeful that knowledge of the mechanism by which fluorescent proteins undergo photo conversion, revealed in this pioneering study, will go a long way to improving imaging and optogenetics used in biological and medical research.
“This work is a tour-de-force in unravelling the molecular level functioning of a light activated protein using the ultrafast time resolved infrared capabilities of CLF-Ultra. The science is driven by the longstanding international collaboration of University of East Anglia (Meech), University of Stonybrook (Tonge), as well as other partners across Europe and Asia"
Paul Donaldson (Central Laser Facility)
The research was supported by the Engineering and Physical Sciences Research Council and the National Science Foundation. The full publication is available to view in Nature Chemistry.
For further information about the research, please contact Professor Peter Tonge (peter.tonge@stonybrook.edu) or Professor Steve Meech (s.meech@uea.ac.uk.)