A recent international collaboration between researchers based at Stellenbosch University, South Africa and the Central Laser Facility at Rutherford Appleton Laboratory saw the use of 2-P-FLIM, available on CLF's OCTOPUS system, to image mitochondrial metabolism in response to protein inhibition. The team, which consisted of Marguerite Blignaut, Barbara Huisamen and Ben Loos from Stellenbosch University (South Africa) and Tony Parker and Stan Botchway at CLF, RAL, exploited the endogenous, naturally fluorescent fluorophore, nicotamide adenine dinucleotide (NAD(P)H) to evaluate changes in NADH usage by mitochondria following inhibition of Ataxia telangiectasia mutated protein kinase (ATM).
The absence of ATM results in an increased cancer risk, neurological degeneration, premature ageing, and is ultimately responsible for the development of the rare disease, Ataxia- telangiectasia. A decrease in ATM protein levels can also increase the risk for insulin resistance and ischaemic heart disease. It has been suggested that dysfunctional mitochondria can contribute to these conditions, as well. Although ATM has been reported in the cytosol of non-dividing neurological cells, this study, published in Scientific Reports, is the first to report the presence of ATM in isolated mitochondria from whole hearts. Mitochondria are often described as the powerhouses of cells, and supply the necessary energy for cells, and the organ they comprise, to function. The heart, which is constantly contracting and relaxing, requires the most energy of all organs and contains a lot of mitochondria to supply it. The researchers found that the inhibition of ATM decreased mitochondrial oxygen usage and energy production when given a carbohydrate-based fuel source which is converted to NADH in mitochondria.
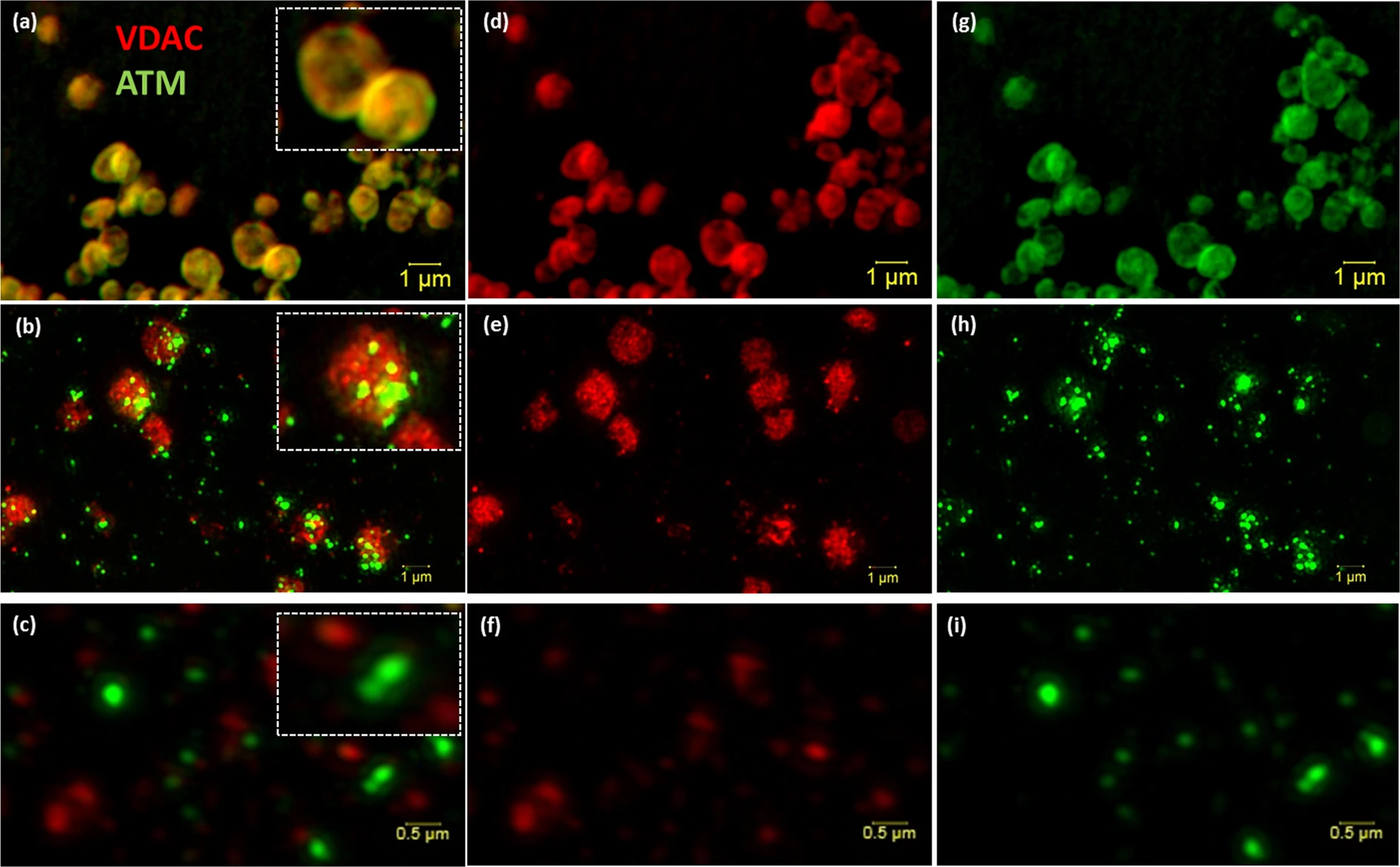
[Super resolution structured illumination microscopy (SR-SIM) of isolated mitochondria and mitoplast preparation. M/S-isolated mitochondria (a, overlay) was probed with VDAC (d; red) and ATM (g; green). (b) (Overlay of e,h) shows the permeabilisation of the outer membrane (e; red) of M/S isolated mitochondria and formation of mitoplasts directly after the addition of 1.2% digitonin (0 min). (c) (Overlay of f,i) represents the formation of mitoplasts after 20 minutes of 1.2% digitonin treatment. Scale bars represent 1 μm in Panel a,b,d,e,g,h and 0.5 μm in (c,f,i).]
Mitochondria produce energy by transferring an electron from NADH through the electron transport chain. NADH is a naturally fluorescent fluorophore that can be detected by fluorescent lifetime imaging microscopy, and allows for label-free imaging of the mitochondrial network. NADH absorbs light at 340 ± 30 nm, which is provided by a laser, and excites it from ground state to excited state. The time it takes to return to its ground state is referred to as decay, and occurs on a nanosecond (a hundred millionth of a second) time scale with the accompanied emission of a fluorescence photon, which can be detected and accurately measured. The near-infra red laser light and use of multiple photon excitation contributes to better resolution and deeper penetration of the samples whilst being less damaging to the samples being imaged. The researchers found that the inhibition of ATM decrease NAD(P)H fluorescence decay lifetime in precursory heart cells, and pinpoints ATM to the mitochondrial machinery required for electron transfer as well as its role in carbohydrate based energy production.
This finding contributes considerably to the results found in South Africa and offers a potential mechanistic explanation for the decrease in energy production. Moreover, a better understanding of ATM's role in mitochondrial metabolism opens up a whole new avenue of research in the cardiovascular and ATM research field, earmarking ATM as a potential drug target and biomarker in disease and treatment progression.
This research has been funded by the CLF-SA Newton Programme.
To read the paper, go here: https://www.nature.com/articles/s41598-019-41108-1
Scientific Image credits and paper DOI https://doi.org/10.1038/s41598-019-41108-1