Paul's interest is in applying the method to commonly used industrial catalysts called zeolites, but the technique is general, and so can find applications across a broad range of chemical problems. This research is part of a wider project funded under his UKRI Fellowship.
Infrared (IR) spectroscopy is a common analysis technique used to study the chemical composition of a sample. It measures the absorption of the IR light by the sample. This can give information about the types of molecules present and their concentrations. In some cases however, it can be difficult to determine concentrations using IR spectroscopy. The strength of IR absorption of a known amount of the compound has to be known. But what if that is not possible? What if even the identity of the molecule is unknown? What if other analytical techniques give ambiguous results? For systems such as complex solids, or complex and transient chemical reaction products, this is can be the case.
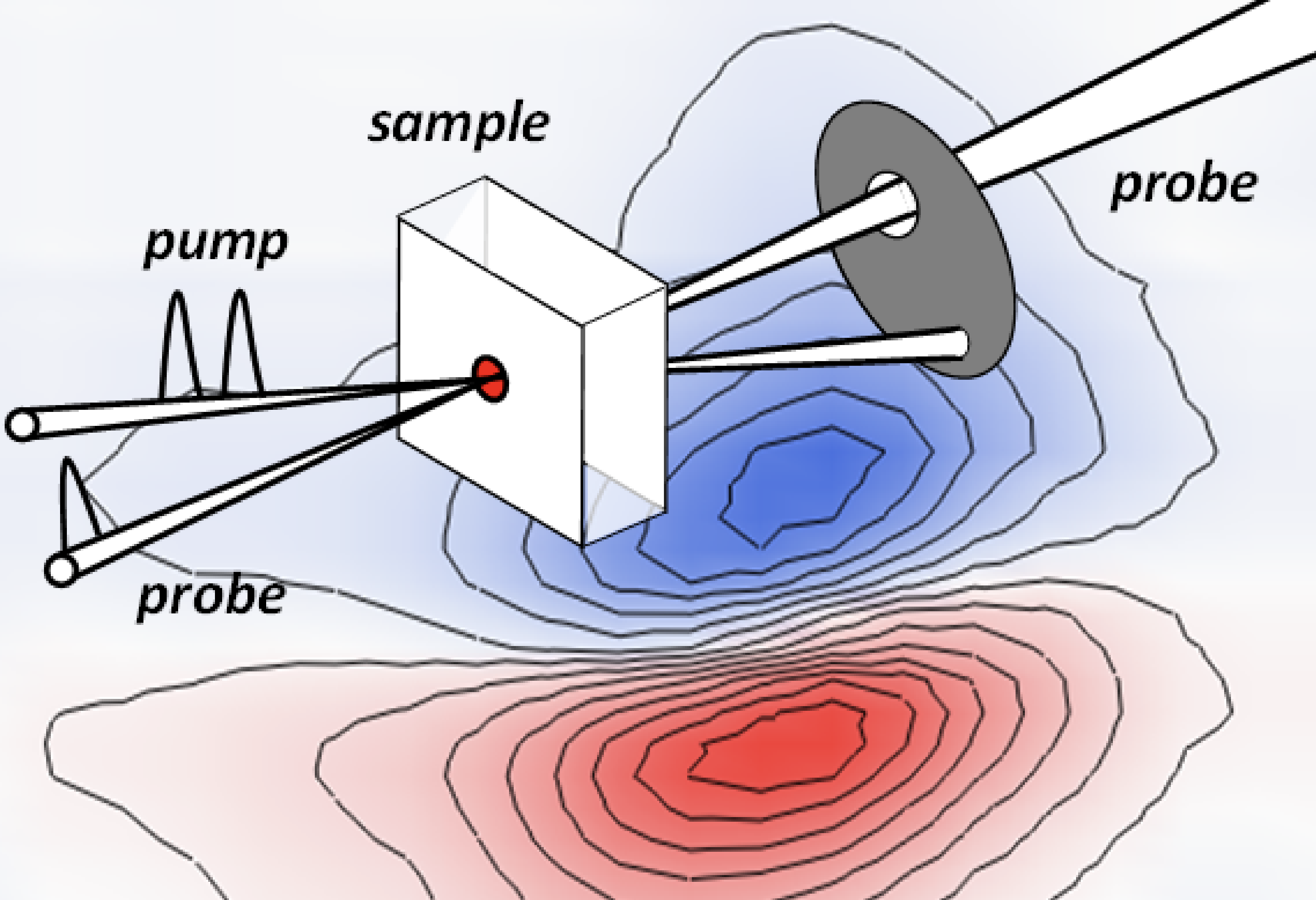
By combining IR absorption measurements with two dimensional infrared (2D-IR) or pump-probe spectroscopy (both IR laser-based techniques are offered at CLF-Ultra), the concentration information missing from IR absorption spectroscopy measurements can be deduced.
Paul worked up this approach to concentration analysis in order to understand IR spectra of zeolites. In fact, the piece of work actually began life as a supplementary information section in a paper about zeolites. However, the approach's novelty and general usefulness meant putting aside the zeolite research to focus on a full paper purely about concentration analysis. The published work shows how to perform the concentration analysis as accurately as possible and tests the approach on simple solutions of molecules to prove its validity.
Paul Explained his process:
“I came up with a related trick about a decade ago to measure the absorption strength of molecules tethered to solutions of gold nanoparticles, without knowing anything about the concentrations of the molecules. This time around, I wanted to know about concentrations of molecules in a sample without knowing anything about their absorption strength – the reverse!
“I did the most basic kind of equation juggling to get a beautifully simple calculation for concentration. It's easy to fall in love with these little discoveries, and easy to go into a mode of promotion of an idea as one's own. But… on writing the manuscript, I dug deep into the literature and saw an awareness of the idea. I found a paper from the 1990s using a very different laser technique for gas analysis long predating 2D-IR spectroscopy. The equations were a little different, but I could see it was the same idea. This paper was published in the journal 'Science', meaning it was considered to be of great importance! What a pity to miss out on that accolade! With the passing of time, the 1990s work did not get noticed, probably because of the uncommon laser technique used. This time around, with IR spectroscopy very common, 2D-IR is becoming more common, and with lots of exciting applications in solution and solid phase chemistry, I hope the idea sticks around!"
By sharing this promising technique, Paul hopes it will benefit the CLF-ULTRA user community, the catalysis community and any analytical chemists out there needing help getting concentration information from their IR spectra.
Read the full paper in Analytical Chemistry here.