A new study published in eLife, has shown that the breast cancer drug lapatinib can sometimes counterintuitively boost the growth of breast cancer cells in the laboratory.
The team responsible for the results was made up of 20 researchers from 13 UK institutions, including CLF scientists Laura Zanetti-Domingues, Marisa Martin-Fernandez, Selene Roberts and Michael Hirsch.
Using microscopes at the Central Laser Facility, the team were able to test clinically-observed, acquired resistance of an anti-cancer drug to targeted HER2 therapy. The group hope that their results will go on to improve targeted therapy against HER2, a receptor protein that is overexpressed in over 20% of breast cancer cases.
HER2 and breast cancer
HER2 is a receptor protein that belongs to the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases. It is found on the surface of cells and is responsible for providing instructions for cell division, combined with outputs from external growth signals. If cells have too many of these receptors, they begin to divide uncontrollably. More division means more growth which can lead to invasive carcinoma - a phenomenon that is witnessed in breast cancer cells. This makes HER2 a key target for therapy.
Traditional treatments for HER2 positive breast cancer work by switching off HER2 receptors and thus inhibiting the proliferation of tumour cells. They do this either by targeting cells from the outside (for example, using monoclonal antibodies like trastuzumab) or from within (e.g. using small molecule kinase inhibitors like Lapatinib). Lapatinib is often used in combination with other cancer drugs and chemotherapy to treat patients with an advanced form of breast cancer. The only problem is that up until now, very little was understood as to why Lapatinib was less successful in clinical trials on its own than the medical community had once hoped.
Scientists now think they have the answer, thanks to a state of the art microscope technique called FRET-FLIM.
“As many breast cancers are triggered by HER2, drugs blocking its action have become cornerstone treatments for these diseases and they've shown great success. But sometimes these treatments can stop working, so there is a pressing need to develop new drugs that can overcome this issue and help improve the outlook for these women."
Dr Justine Alford (Cancer Research, UK)
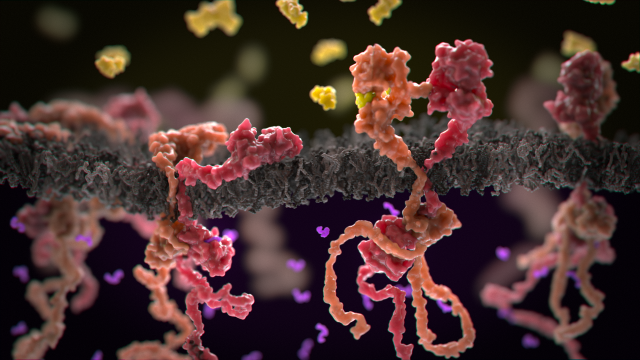
Figure 1: Inactive (left) and active (right) HER2-HER3 pairs. Image credit – Phospho Biomedical Animation.
FRET-FLIM microscopy combines the power of FLIM (Fluorescence lifetime imaging microscopy) with FRET (Förster resonance energy transfer) to provide an imaging technique with extremely high spatial and temporal resolution. This means that scientists can paint a beautiful picture of the way that proteins interact in cells.
Lapatinib works by promoting the formation of - and stabilising a heterodimer of - the cell membrane receptor protein HER2 and its pseudokinase partner HER3. This dimer can then drive unnatural liaisons with the HER3 ligand neuregulin - an extracellular growth factor commonly associated with breast cancer- resulting in the proliferation of tumour cells. Using FRET-FLIM microscopy, the sub-team at Kings College London were able to highlight not only the complex role that protein conformation plays in acquired resistance to lapatinib, but also the problems one faces when targeting kinases with ATP-competitive inhibitors.
Whilst FRET-FLIM was ideal to study the pairs of mixed HER2 and HER3 receptors, scientists also used the OCTOPUS super resolution microscopes to show that these receptors can function in groups and this may prime the molecules for activation. Stochastic optical reconstruction microscopy (STORM) imaging at the CLF showed that drug treatments also cluster these receptors, potentially resulting in the intensified effect of ligand binding.
“To understand the mechanism by which these receptors function in a cellular environment requires quantitative, nanoscale imaging. This study makes it clear that such analysis is imperative for future drug design and safety testing. There is a wealth of detail yet to be revealed from the many signalling systems needed to maintain the health of our cells."
Dr Selene Roberts (Central Laser Facility)
The paper - published in the journal elife – presents evidence for the synergistic behaviour of the HER3 ligand neuregulin and the breast cancer drug lapatinib being dependent upon HER3's ability to bind ATP.
Their data showed that stabilisation of the HER3 kinase domain with an ATP-competitive kinase inhibitor can actually have a stimulating effect on HER2+ breast cancer cell proliferation. The team therefore provide solid evidence to support the assumption that future developments of targeted therapy should be focused towards the synergistic proliferative effects of lapatinib and neuregulin, as oppose to the stabilisation of the HER3 ATP binding pocket, as suggested in previous studies.
“Drug resistance remains a major obstacle to effective targeted therapy in many cancers, including HER2 positive breast cancer, the subject of our current study. This paper builds on our growing understanding of how some drugs can paradoxically activate their intended targets, which can lead to drug resistance. This work will allow us to design more effective HER2:HER3 targeted treatments in the future"
.
Co-senior author Dr Angus Cameron (Queen Mary University of London)
“In recent patient studies, HER2 targeted therapies that combined lapatinib with the antibody treatment trastuzumab successfully controlled HER2 positive breast cancers at first, but did not improve longer term disease-free survival. Our new findings could help us design future studies to improve combined HER2 targeted therapies."
Professor Tony Ng (School of Cancer and Pharmaceutical Sciences at KCL)
The research was supported by Cancer Research UK, the BBSRC, Dimbleby Cancer Care, KCL-UCL Comprehensive Cancer Imaging Centre and in association with the DoH, the Medical Research Council, and the Swiss National Science Foundation.
The full publication is available to view in eLife.
For further information about the research, please contact Dr Angus Cameron (a.cameron@qmul.ac.uk) , Professor Franca Fraternali (franca.fraternali@kcl.ac.uk) , Professor Tony Ng (tony.ng@kcl.ac.uk) or Professor Peter J Parker (peter.parker@crick.ac.uk) .
