Carbon is extremely versatile and can form many different molecular structures called allotropes. For example, both graphite and diamond are carbon allotropes, but due to the arrangement of their carbon atoms, they are very different from one another.
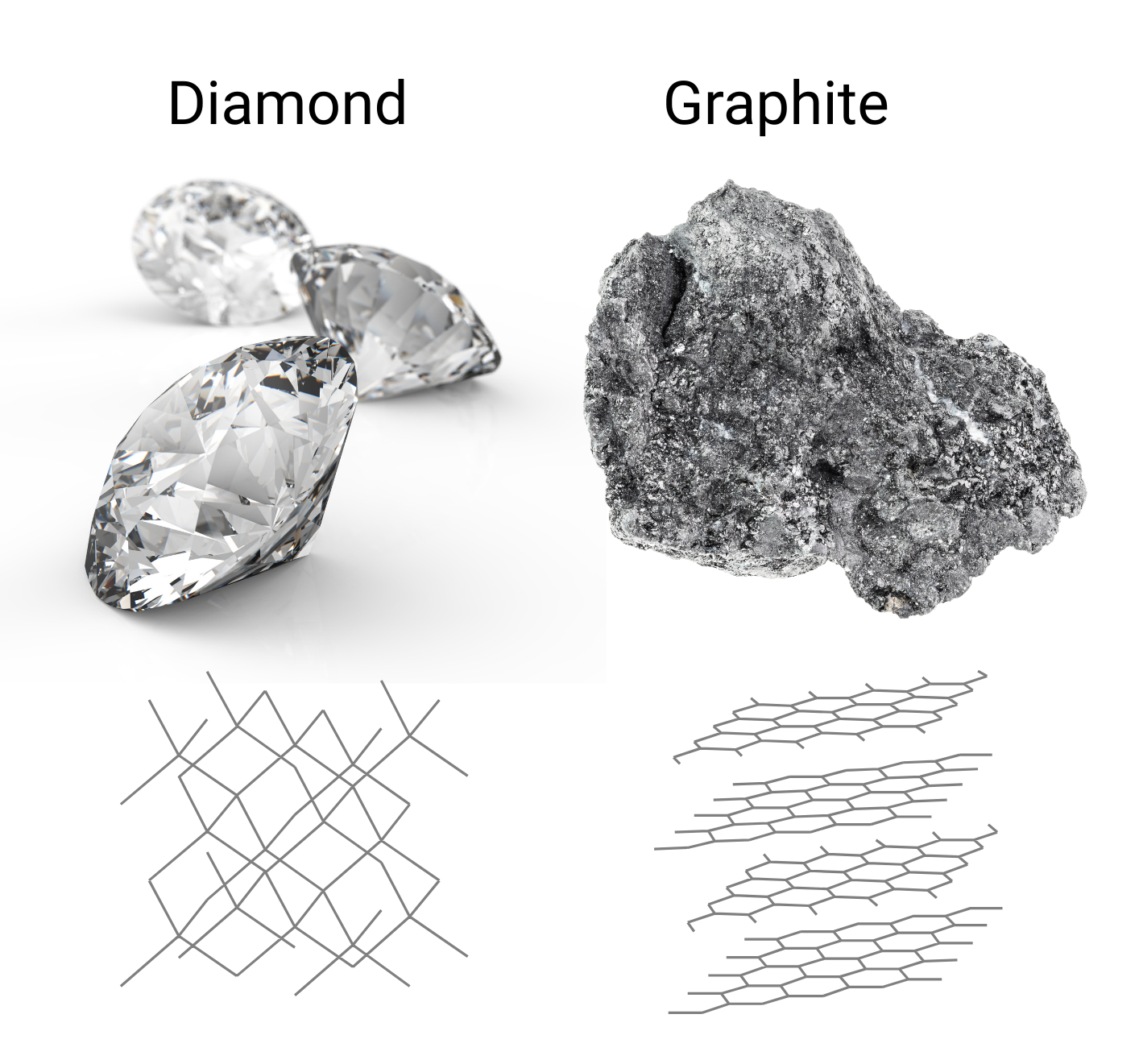
Diamond is one of the hardest minerals, used in drilling and cutting, whereas graphite is soft enough to use in everyday pencils.
Ring-shaped carbon molecules, called cyclocarbons, show potential as candidates for atomic-scale electronic wires and circuits which could make them useful as electronic materials. However, linear chains of carbon atoms, like these cyclocarbons, are notoriously reactive and unstable at room-temperature, making them difficult to create and study.
Oxford's Chemistry group, who collaborated with the Central Laser Facility (CLF), the University of Manchester and the University of Bristol, published their achievement on Thursday 14th August in Science.
After years of work, the team led by Prof. Harry Anderson managed to stabilise a ring of 48 carbon atoms by threading it like a string through three smaller rings. These smaller rings helped protect the larger carbon ring from reacting with the environment and breaking apart. With a half-life of nearly four days, the ring survived long enough to be studied using Mass Spectroscopy, Nuclear Magnetic Resonance (NMR), and Raman Spectroscopy.
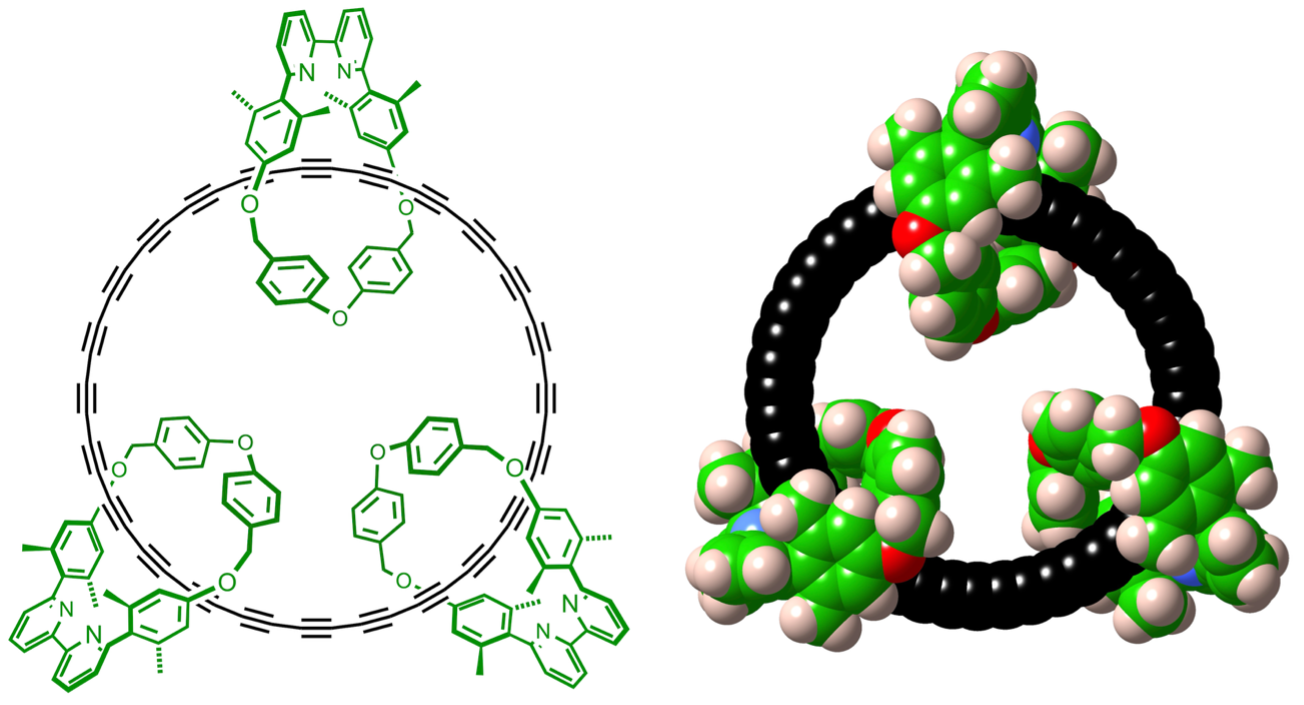
Left: Chemical structure of the cyclocarbon catenane made by the team. Right: Space-filling representation. Catenanes are the term used for a molecule made of two or more interlocking rings. Image Source: https://doi.org/10.1126/science.ady6054
Lead author Dr Yueze Gao (Department of Chemistry, University of Oxford) said: “Achieving stable cyclocarbons in a vial at ambient conditions is a fundamental step. This will make it easier to study their reactivity and properties under normal laboratory conditions."
Study senior author Professor Harry Andersen (Department of Chemistry, University of Oxford) said: “This achievement marks the culmination of a long endeavour to synthesise cyclocarbon catenanes, based on the hope that they might be stable enough to study at room temperature... It is satisfying to have reached this point, because there were many times when the goal seemed unrealistic and unachievable…"
The CLF is incredibly proud to have been entrusted to study these these groundbreaking molecules with our Raman instrument.
Raman Spectroscopy can be useful for identifying molecules, even with differences as subtle as allotropes, due to its ability to 'fingerprint' molecules for identification. It does this by bouncing a laser light off a molecule, causing it to vibrate in a way that is unique to each molecule. The result is that the laser light returns a different colour, having had some of its energy taken by the molecule. The CLF has a suite of different Raman instruments, giving us the potential to study even the molecules that elude conventional Raman.
Read the University of Oxford press release here.
Read the Science paper here.
*Read the buckminsterfullerene 'buckyball' paper here.
