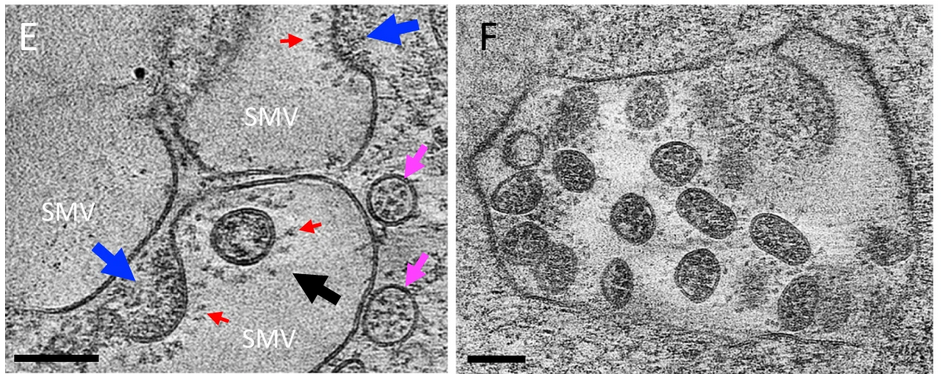
E. An enlarged view (at a different angle) of boxed area in C,
showing assembled virus (black arrow), assembling viruses (blue
arrows), spikes (red arrows), and spike-containing vesicles (pink
arrows). F. Large intracellular virus-containing vesicle (LVCV) full of readily assembled viruses.. https://www.nature.com/articles/s41467-021-24887-y/figures/3
Researchers from the Central Laser Facility, Diamond, University of Pittsburgh, University of Oxford, and University of Cambridge spearheaded research on the replication cycle of the SARS-CoV2 virus. The Nature Communications publication is the first study to reveal structural and ultrastructural information on the virus's replication cycle in close to real-life frozen conditions.
Because of the severity of the situation last year, researchers from CLF's OCTOPUS Imaging Cluster were among the first to receive rapid access at the facilities to study the virus. As there was a consortium of researchers involved in the study, different multi-modal imaging techniques were used to extract as much information about the virus as possible.
One of the techniques used to probe the virus was the cryo-FIB/SEM at OCTOPUS. An optimised work set up from a previous investigation that looked at Leigh Syndrome was used, where CLF scientists sliced parts of the cell using a focus ion beam (FIB) and used a scanning electron microscope (SEM) to take 2D images of the frozen cells. These sections of the cell were then reconstructed to form 3D images. The 3D image of the infected cells demonstrated that COVID-19 had visible structural alterations in them, like extensive membrane tunnels formed for the virus to exit and lesions near the exit region of the cell. To get an in-depth perspective of what's going on in the macromolecular complexes of an infected cell another imaging technique called cryo-ET was used. Combined together, these provide a complementary view of the virus's activity.
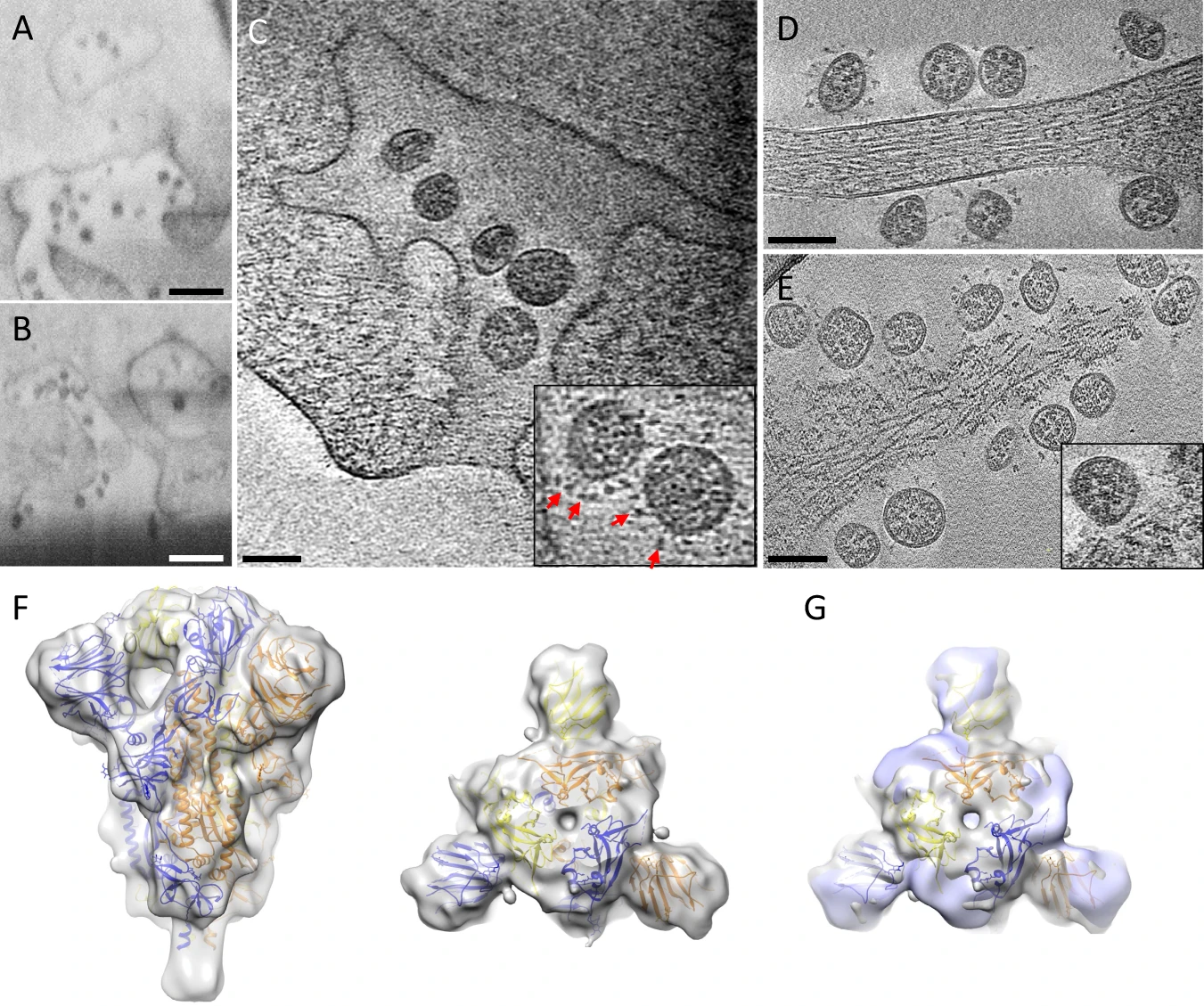
A, B CryoFIB/SEM images of cell periphery, depicting virus
particles exiting through extended tunnels connected to external of the
cell. C CryoET of the SARS-CoV-2-exiting tunnel. Inset shows a close-up view of viruses with prefusion spikes (red arrows). D, E Viruses released from the cell, close to an intact cell membrane (D) or next to membrane lesion sites (E). Inset, close-up view of a membrane lesion site. F Subtomogram average of spikes on released viruses at 10.6 Å resolution fitted with an atomic model of spike trimer (PDB 6VXX)16, viewed from side and top. G
Comparison of spike structures from intracellular assembled viruses
(transparent blue) and extracellular released viruses (transparent
gray), viewed from top. Scale bar is 300 nm in A, B and 100 nm in C–E. https://www.nature.com/articles/s41467-021-24887-y/figures/5
Cryo FIB/SEM and cryoET revealed that the virus replicates in a highly organised and efficient manner. These were the steps of the virus replication cycle:
- RNA Synthesis and Transport: The first step is replication of the viral RNA. The replication takes place in an enclosed space called a double membrane vesicle (DMV). Viral RNA (vRNA) is packaged into a DMV, so it evades a patient's immune system
- Assembly and Budding: vRNA being produced pushes other structural proteins to be formed (ex. N,M,E and S proteins) at the protein production site. The virus spikes, which give the virus its signature shape are also produced at this site
- Assembly and Budding: Structural proteins, spikes and vRNA fuse together and are enclosed in a space referred to as a single membrane vesicle. Through further production of the structural elements, single membrane vesicles containing the virus become large virus contained vesicles.
Virus egress: These large virus containing vesicles can exit the cell through the membrane tunnels and release viral content that then infect other cells
RNA synthesis, assembly, budding and egress are all common steps through which all viruses evade our immune systems. This study has demonstrated that when multi-modal imaging techniques like cryo FIB/SEM and cryoET are combined, we not only get a detailed image of what happens to a whole cell but also what goes on within individual molecules present in the virus at near-native state. Furthermore, this imaging study of COVID-19 emphasizes the potential these combined techniques can have to study other diseases in greater detail.
Head of Octopus Facility, Professor Marisa Martin-Fernandez said, "This is a great example of how the large-scale facilities on the Harwell campus can work together on scientific problems that are important for all of us. It's been a real team effort, and I'm delighted to have played a part."
Further information:
Full paper can be found here.
