A discovery that has
shown how plants may defend themselves in the face of pathogen attacks
could hold the key to making crops more disease-resistant.
As part of a BBSRC-funded project led by Oxford Brookes University, STFC’s Central Laser Facility has developed a unique technique that has answered a question which has puzzled scientists for many years – why certain proteins in plant cells don’t move around as much as their counterparts in animal cells.
By enabling the movement of individual molecules in living plant cells to be observed in real time for the first time, the new technique has revealed that the cell wall plays a crucial role in limiting the mobility of proteins produced when a plant comes under attack. Specifically, it has shown that the cell wall allows these proteins to stabilise in the plasma membrane . This restricts their ability to move around and fight invadin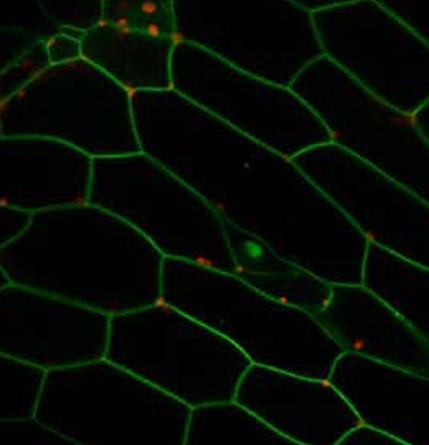 g pathogens and so increases the plant’s vulnerability.
g pathogens and so increases the plant’s vulnerability.
References
- Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Martinière A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta-Peyret L, Luu DT, Botchway SW, Webb SE, Mongrand S, Maurel C, Martin-Fernandez ML, Kleine-Vehn J, Friml J, Moreau P, Runions J. Proc Natl Acad Sci U S A. 2012 Jul 31;109 (31):12805-10. doi: 10.1073/pnas.1202040109 (link opens in a new window)
- CLF contact: Stan Botchway, email
Plant membrane proteins anchored at the plasma membrane
(Credit: Dr John Runions, Oxford Brookes University)
Researchers in the Indian Ocean realised that clouds drifting downwind of cities had a different level of reflectivity. It is believed that air pollution affects how reflective clouds are, as surfactant films can enclose water droplets with an oily layer and prevent them growing to form raindrop lets or shrinking to evaporate.
Clouds are formed when a large mass of air moves upwards in the atmosphere, and cools. Water vapour condenses to form droplets, each one formed around a particle and the whole cloud dynamic can be changed by what’s in the droplets. However, everything that is released into the atmosphere is oxidised. The atmosphere acts, in effect, like a low-temperature combustion system, and the surfactant films are short-lived. They oxidise with ozone found naturally in the atmosphere, freeing the cloud droplet to expand or contract according to local conditions.
A team from Royal Holloway University of London working with CLF scientists have been investigating the rates of different chemical reactions that remove these surfactant films, studying the chemistry which is so important in the atmosphere. To do so they used complementary studies at the CLF, Diamond Light Source and the ISIS Neutron and Muon source. The team used the CLF’s optical laser trap system (located in the Research Complex at Harwell) to hold a water droplet in the focus of a laser. This allowed the surface chemistry of the droplet to be analysed using spectroscopy techniques. The droplets, which are doped with a small amount of pollutant material, were then exposed to ozone to model atmospheric reaction processes, and changes in their surface chemistry during the reaction were measured bioanalysis of the spectra. Neutron investigations allowed the team to measure the rate of loss of the surfactant film by monitoring the reflectivity of the droplet during chemical reactions. Determination of these properties provides greater understanding of cloud droplet behaviour in the atmosphere, and its impact on the climate.
References
- Jones, S. H., King, M. D. and Ward, A. D., Atmospherically relevant core–shell aerosol studied using optical trapping and Mie scattering, Chem. Commun., 2015, 51, 4914—4917. DOI: 10.1039/C4CC09835H (link opens in a new window)
- Hunt, O., Ward, A. D. and King M.D., Heterogeneous oxidation of nitrite anion by gas phase ozone in an aqueous droplet levitated by laser tweezers (optical trap): Is there any evidence for enhanced surface reaction? Physical Chemistry Chemical Physics. 14, 4, p. 2734 – 2741, 2015. doi: 10.1039/c4cp05062b (link opens in a new window)
- CLF contact: Andy Ward, email
A research group from the STFC Central Laser Facility and the University of Manchester have been looking at the bio-accumulation of radioactive material by bacteria in soil.
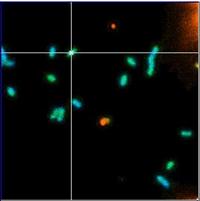 Targeting bacteria: Two-photon fluorescence confocal microscopy images Geobacter sulfureduccens containing uranyl acetate
Targeting bacteria: Two-photon fluorescence confocal microscopy images Geobacter sulfureduccens containing uranyl acetate
(Credit: STFC)
Knowledge of the long-term storage, environmental behaviour and mobility of waste radioactive material associated with disposal has become imperative with respect to the UK’s energy strategy. At RAL, a team of scientists from Manchester University, headed by Dr Louise Natrajan, is using facilities at the CLF to investigate how microbes can help prevent radioactive waste materials from spreading in soils.
Certain microorganisms, such as bacteria, fungi and algae can bind and accumulate radioactive material through biosorption. Indeed, there are some strains of bacteria associated with soils that are capable of facilitating a chemical reaction whereby the radioactive material no longer dissolves in water. Such behaviour can markedly alter transport of this waste in the environment.
Using imaging techniques at the CLF, the team is monitoring the properties of uranium compounds in bacteria to provide fundamentally important information on both the micro-environment and chemical reaction processes. Laser beams are used to target individual bacteria that are imaged using techniques called fluorescence lifetime and phosphorescence lifetime imaging. Their studies are revealing the location and chemical changes in the microbial colony.
References - Fluorescence spectroscopy and microscopy as tools for monitoring redox transformations of uranium in biological systems, Debbie L. Jones,Michael B. Andrews, Adam N. Swinburne, Stanley W. Botchway, Andrew D. Ward, Jonathan R. Lloyd and Louise S. Natrajan, Chem. Sci., 2015, 6, 5133-5138. DOI: 10.1039/C5SC00661A (link opens in a new window)
- CLF contact: Andy Ward, email
