Studying the intricate processes inside cells is crucial to understand how they operate and their function in the wider body. It also allows scientists to investigate the early stages of diseases that originate within the cells themselves, such as cancers. However, it is difficult to probe into tiny, delicate cells without damaging or otherwise affecting them, and consequently distorting any results. Fortunately, one technique that helps scientists to non-invasively see cellular function is fluorescence microscopy.
Something that is fluorescent absorbs a certain colour of light and in return emits another almost immediately. To an observer, this appears to light up the region of interest. We can give a sample (or even just a particularly interesting part of it) this property through special dyes or coatings, which helps us to view and image it. But when it comes to studying cells, we need to be careful what this coating is. Firstly, it needs to be stable, which means it won’t interact too much with the cell and make it behave differently to how it would in the body. It also cannot be significantly toxic to the cell, especially if we want to image live samples. Nanoparticles are a great contender for such a coating – they are tiny, which lets the cells uptake them, and many are unreactive and undamaging. Fluorescent nanoparticles are therefore excellent at highlighting specific areas that scientists wish to explore and tracking movements within a sample.
A research team comprising of the CLF together with the University of Cambridge, the Chinese Academy of Sciences, Diamond Light Source, ThermoFisher Scientific, and The Faraday Institution have now used the CLF’s Octopus facility to investigate a new class of ultra-small nanoparticles which may be used in fluorescence imaging. They absorb and emit light through a process known as ‘Triplet-Triplet Annihilation Upconversion’, or TTA-UC.
TTA-UC is particularly useful because it overcomes a problem that is encountered in some fluorescence microscopy techniques. It is possible for a sample to have its own natural autofluorescence, and this can sometimes overshadow any ‘human-made’ fluorescence that has been applied to study a particular component or area of that sample. The TTA-UC process emits light less frequently and at different orders of magnitude time-scales compared to natural processes, and so its light can be easily identified and picked out from the crowd due to its long lifetime. It’s also highly efficient in its light emission - so much so that the nanoparticles studied at Octopus have been suggested for applications in light emitting devices or making solar cells to produce electricity.
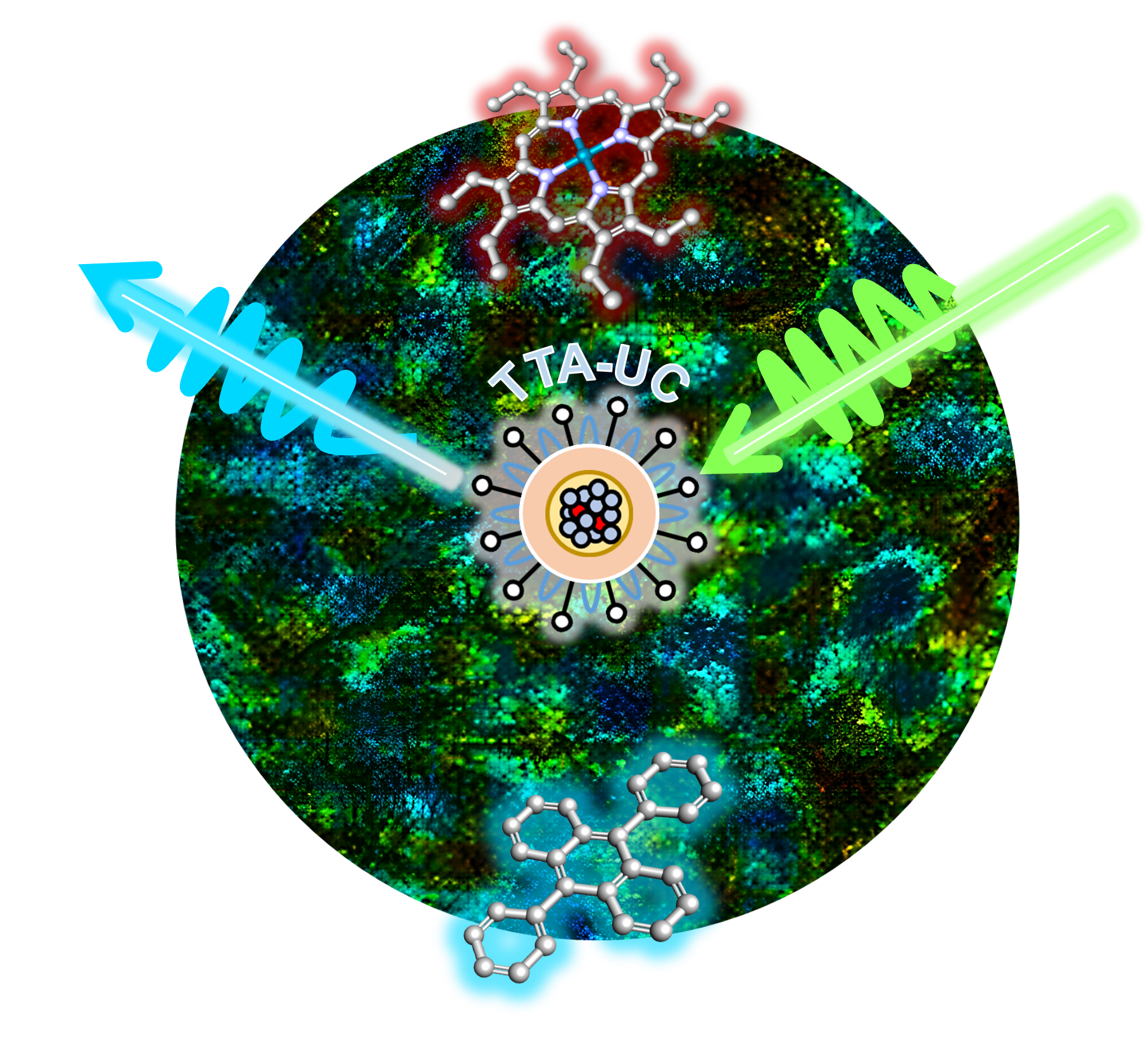
Aside from their fluorescence, the team’s nanoparticles have many qualities that would make them perfect for bioimaging. Compared to other materials they are less likely to interact with oxygen molecules, which can inhibit the emission of light. Their light emission lifetime is also well-balanced – long enough for the light to be distinguishable, while not too long as to risk cellular damage.
These TTA-UC nanoparticles also have one more trick up their sleeve. Scientists can observe fluctuations and changes in the light they emit from inside biological cells, which gives them a new level of detail. In particular, they can highlight the movement of oxygen throughout the cell so it can be mapped to tell us more about what is happening in each area. In the future, the sensitivity of the nanoparticles to other variables such as pH and temperature could be investigated to tell us even more about how cells react and function, helping us to better understand the complex processes inside the body and how we can adapt our treatments for disease.
Prof. Stan Botchway explains, "It is essential that chemists continue to develop new chemical probes for biological and medical applications. When combined with advanced imaging platforms such as phosphorescence lifetime imaging microscopy (PLIM) as is the case here, we are able to gain significantly more information from cellular processes than simply imaging the fluorescence steady state."
