In collaboration with the OCTOPUS team, Imogen has been using the techniques of fluorescence lifetime imaging and Total Internal Reflection Fluorescence microscopy combined with optical trapping to study the inner working of plant cells. The latest work has recently been published in Communications Biology.
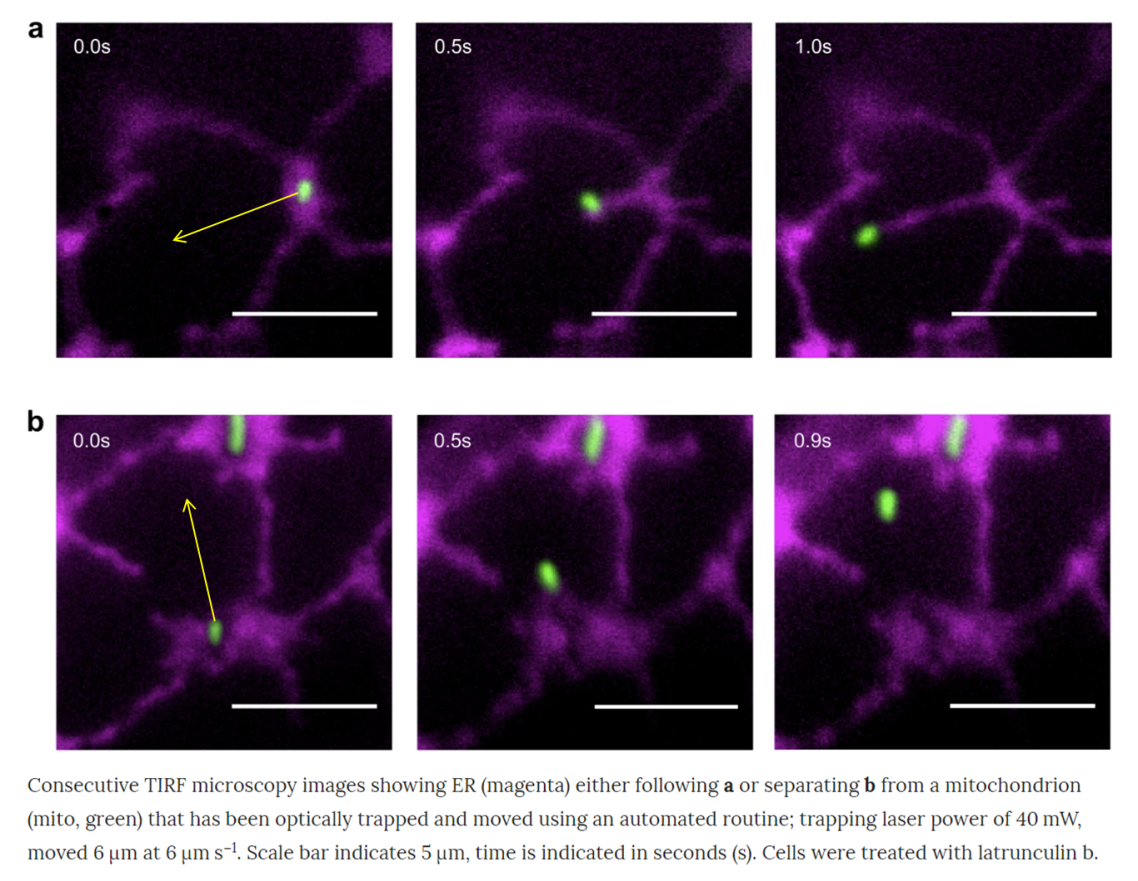 Videos and Figure credited to Figure 1 of White, R.R., Lin, C., Leaves, I. et al. Miro2 tethers the ER to mitochondria to promote mitochondrial fusion in tobacco leaf epidermal cells. Commun Biol 3, 161 (2020). https://doi.org/10.1038/s42003-020-0872-x.
Videos and Figure credited to Figure 1 of White, R.R., Lin, C., Leaves, I. et al. Miro2 tethers the ER to mitochondria to promote mitochondrial fusion in tobacco leaf epidermal cells. Commun Biol 3, 161 (2020). https://doi.org/10.1038/s42003-020-0872-x.
In the recent paper, we show that mitochondria, organelles that supply energy in the cell, are directly tethered to the endoplasmic reticulum (ER) in leaf epidermal cells, and can be regulated by the GTPase protein AtMiro2. Although mitochondria and ER association has been inferred previously, we now provide evidence of a physical interaction via optical tweezers. Using the same technology, we have shown other organelles physically interact.
But why do organelles need to physically tether themselves to one another?
The cell can be viewed as an island: For an island to 'work' efficiently it requires goods to be transported and exchanged across the land. Here, in a cell, similar processes are occurring with organelles transporting 'goods' to enable the cell to maintain itself. This process may require organelles to exchange components or may in fact enable organelles themselves to multiply.
In our recent work, we show that the functional role of mitochondria tethering to the ER is to enable mitochondria to fuse. Mitochondria can undergo rounds of fission and fusion to maintain the level of mitochondria within the cell. Our findings indicate the first molecular component, Miro2, which could affect mitochondria fusion, a process which requires interaction with the ER.
Using optical tweezers, our future work will expand our understanding of the functional role conveyed through organelle interactions in subcellular communication. This is vitally important if we are to develop novel ways in which to alter plant growth and responses to pathogens to enable changes in production of food and plant based products.
To view the paper, click here.
