Unsurprisingly, this old practice has become artfully well developed over the years. Any chemist can tell you that if you take, for example, carbon dioxide, you can turn it into carbon monoxide using electrochemistry. However, it is still unclear what processes are actually going on right at the surface of the electrode when this happens.
There are experimental methods that can detect the miniscule, thin layers of surface molecules amidst the overwhelming quantities of non-surface molecules. These are readily applied to materials such as water or water-glass interfaces, yet it remains that very few experiments have been done that apply these methods to electrochemistry – despite there being a need for it in this area of research.
However, last year a team of 3 scientists from the University of Liverpool and the Central Laser Facility (Dr Alex Cowan (Liverpool), Dr Gaia Neri (Liverpool) and Dr Paul Donaldson (CLF)) used the Ultra laser facility to take a laser technique which is commonly used for surface observation and applied it to electrochemistry – a seemingly simple idea, but, if successful, would be a huge benefit to the electrochemistry community.
Using Ultra to make molecular level observations with the laser technique called IR-Visible vibrational sum frequency generation (VSFG), the technical outcome of this experiment was to observe the comings and goings of electrocatalyst molecules at metal electrodes and to find out why gold was better than platinum for an example of the CO2 to CO chemical reaction.
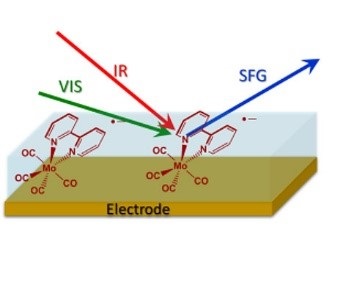
Put simply, VSFG is a technique where short IR and visible laser pulses are overlapped spatially and temporally at a sample surface. When the IR laser frequency matches that of a surface molecule's vibrational mode, the vibrationally polarized molecules can interact with visible light via their electron clouds, resulting in the generation of light at the sum of the incident IR and visible frequencies.
This technique provides the opportunity to gain new insights into the chemistry and stability of species at electrode surfaces that are not readily obtainable by conventional methods such as FTIR spectroscopy.
Via this approach, the team was able to report an in situ VSFG study on the electrocatalytic reduction of CO2 using [Mo(bpy)(CO)4] as the catalyst. During these cyclic voltammetry (CV) experiments, VSFG gave clear spectroscopic evidence of the nature of the intermediates formed on the electrode surface and report on the nature of the electrode-catalyst interactions with both gold and platinum. Dr Cowan commented: “To the best of our knowledge this represents the first in situ study of a molecular electrocatalyst under potentiostatic control by VSFG spectroscopy."
In addition to these results, it is clear that technique will be a beneficial tool for the community because it shows that VSFG measurements conducted by the team are a powerful way of revealing the reactions of catalysts at electrodes; in this case showing exactly how control of the electrode–catalyst interaction offers an alternative route to unlocking the potential of this under-studied class of CO2 reduction catalysts.
The scientific community has recognised these important findings, and as a result we at the CLF are proud to share that the paper has been published in the JACS Young Investigators Virtual Issue.
Authors Drs Cowan, Donaldson and Neri are over the moon. Paul stated, “It's been a fascinating and very rewarding collaboration between Alex and Gaia (electrocatalysis experts) and myself (CLF, spectroscopy). It is great to see that the measurements we made towards the JACS paper have been recognised as making a really useful contribution in the field!"
On the online publication, Associate Editor Klaas Waynne stated, “This exciting demonstration opens the way to mechanistic studies of electrochemistry using VSFG, which have been much neglected but are sorely needed by the electrochemistry community."
To read find out more, please go to: https://pubs.acs.org/doi/10.1021/jacs.7b06898
