A team of researchers from the University of Kent and the University of Essex recently published a research article about their investigations into the localisation and function of two class 1 myosins in the nematode worm C. elegans. The article was published in the cell biology journal Cytoskeleton.
In this study, the scientists sought to uncover the localization and function of two myosins, HUM-1 and HUM-5, in the popular model organism C. elegans. Using Confocal laser scanning microscopy (CLSM) and the Structured Illumination Microscopy (SIM), with the SIM data acquired via the OCTOPUS imaging cluster at the Central Laser Facility, the team was able to image where the two fluorescently labelled myosins were expressed in C. elegans. They found that HUM-1 is found in a wide range cells and tissues (Fig 1) while HUM-5 is exclusively expressed in a specific type of cells in the nervous system (Fig 2).
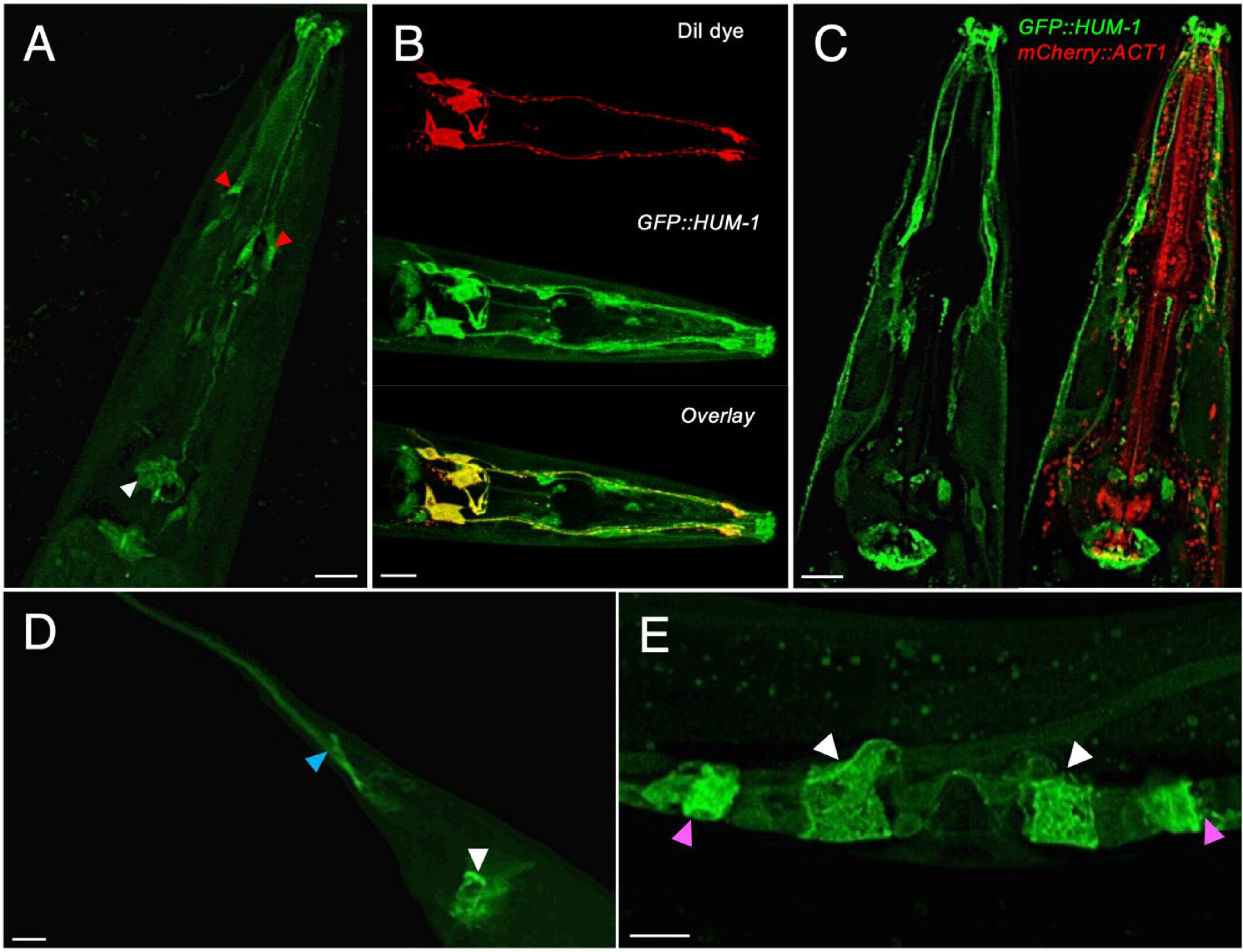
Fig 1. Location of HUM-1 in worms shown in fluorescent green. (Image credit: https://doi.org/10.1002/cm.22026)

By studying and comparing mutant C. elegans, they also found that the nematodes produced significantly fewer offspring when they lack HUM-1, confirming HUM-1's role in reproduction. Furthermore, nematodes lacking both HUM-1 and HUM-5 were observed to have a shortened lifespan, suggesting that the combined effect of both myosins play an important role in lifespan regulation.
Finally, they discovered that the phosphorylation of, or the attachment of a phosphate group (PO43-) to, HUM-1 affects both its localization and function. Their finding indicates that phosphorylation plays a role in regulating the interaction of HUM-1 with the cell surface membrane. Interestingly, this finding was made not just in C. elegans but also in the fission yeast, S. pombe (Fig 3). Based on this, the team concluded that the finding is applicable to all eukaryotes, including plants, animals, fungi, and protists.
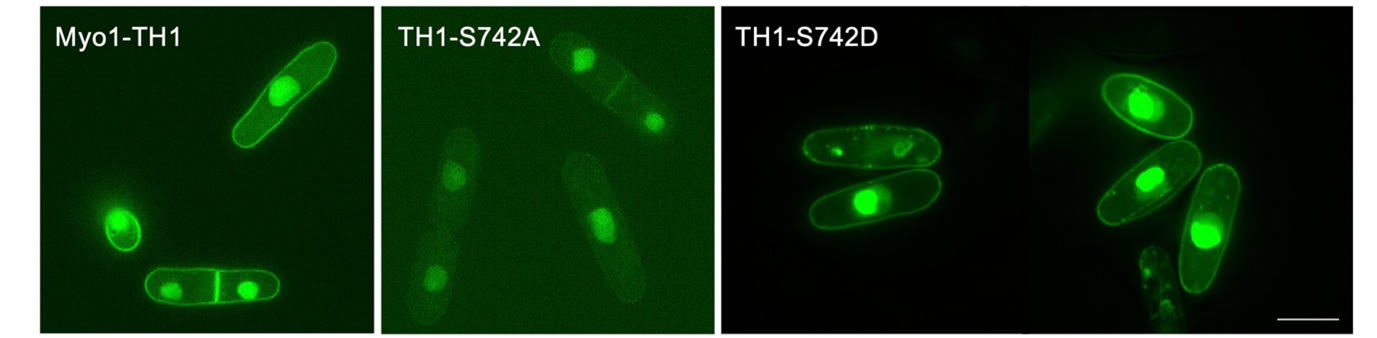
Fig 3. Location of Myo1 shown in fluorescent green. Scale bar: 10 μm. (Image credit:https://doi.org/10.1002/cm.22026)
Class I myosins are motor proteins which, alongside other proteins, bind with another type of protein called actin to form the actin cytoskeleton. Besides providing structural support, the actin cytoskeleton is also a highly dynamic system that plays a crucial role in various cellular processes such as motility, intracellular transport, cell growth and cell division. Therefore, understanding the localisation and function of myosins can help us better understand how cells function in general, laying a crucial foundation for broader biological research.
Find the research article here.
