(Illustration by Helen Towrie, CLF, STFC)
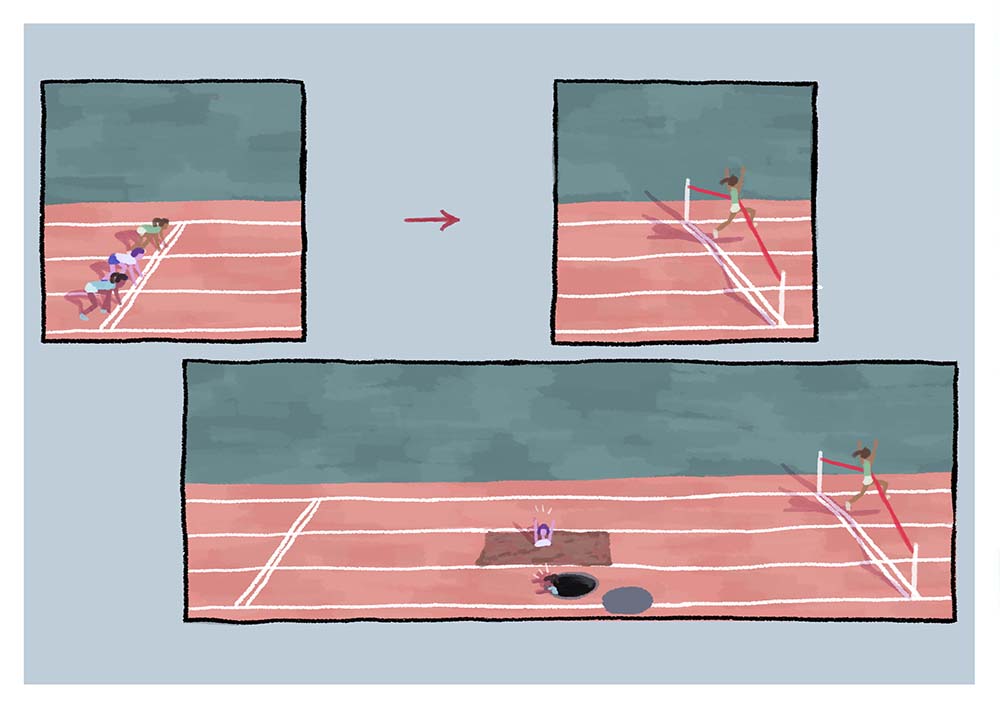
Picture an Olympic level race, held in a stadium where a box has been built over the track so that the only visible parts are start and finish lines. The athletes leave the starting blocks, nothing can be seen for a while, and then the crowd roars as a clear winner crosses the finish line. Would you be satisfied with this outcome? Would you trust that there was no foul play during the time the athletes weren't visible?
This is what observing a complex photochemical reaction is like, though instead of a box covering the process, it is simply that key parts of the reaction cannot be measured using most spectroscopic techniques.
However, using Artemis laser, a team from University of Southampton in collaboration with scientists at the CLF have been working to try to uncover what really happens during these extremely fast timescales. Photochemical processes involve complex coupled motion of electrons and nuclei on fast timescales, leading to a dynamic flow of energy between nuclear and electronic degrees of freedom and, through competing processes, to the eventual formation of reaction products.
The team's experiment involved probing the dynamics of dissociating carbon disulphide molecules (CS2) across the entire reaction pathway upon UV excitation thanks to Artemis' high-harmonic XUV pulses.
Using this method, Russell Minns and his team managed to extend the observation window in gas-phase photoelectron spectroscopy by using a 20eV XUV probe, which allowed them to observe electrons from all excited states and final products and work out the photodissocation pathway in CS2. This achievement has been recognised and published in the latest edition of Physical Review Letters.


(Dr. Russell Minns standing next to Artemis)
Russell reflected on the experiment's origins, remembering how the first tiny success led to this, “We initially started doing a version of this experiment way back in 2011 – We were trying to do XUV photoelectron spectroscopy. Myself and the team collected data for about 4 hours, and then on one of our readings we saw a tiny peak – and that was the start of it."
Russell went on to explain that this experiment is a step towards studying larger more complex molecules in the future.
“The experiment itself is sort of a “proof of principal" experiment, what we are trying to do is develop techniques that allow us to monitor the entire reaction so that we can get a proper experimental measure of the mechanisms of these things.
“I've been working with the Artemis team on and off for 6-7 years now – it's always good to come back here as there's a real collaborative atmosphere," Russell continued.

(Dr. Russell Minns with PhD Student who led the data analysis of the experiment, Emily Warne)
“In regards to the future, this experiment has the potential to help us gets to grips with the mechanisms of chemical dynamics. Currently, we have many different models that help us describe the reaction process, but in almost all cases, they have never have a direct measure of the transition states and the structures that molecules go through."
This experiment shows a way to lift the cover off the track and watch the whole of the race.
To discover the full details of this experiment, please follow this link: https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.120.183003
Experiment Leader: Russell Minns
Theory provided by: Adam Kirrander
Proposal PI and experiment link scientist: Richard Chapman
